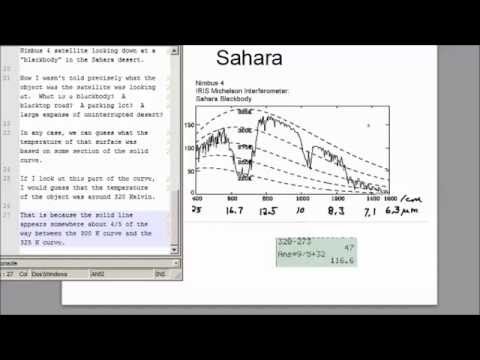
Wavelength to Wavenumber: The Complete Conversion Guide
Spectroscopy, a cornerstone of analytical chemistry, relies heavily on the relationship between a wave's energy and its properties. The conversion process, wavelength to wavenumber, represents a fundamental operation in fields utilizing techniques like Fourier-Transform Infrared (FTIR) spectroscopy. NIST (National Institute of Standards and Technology) provides crucial reference data for spectral analysis, underscoring the importance of accurate wavelength to wavenumber transformations. These transformations are indispensable for researchers across various disciplines to understand the underlying properties of matter and this conversion unlocks deeper insights into light-matter interactions.
Image taken from the YouTube channel Najam Academy , from the video titled Wavenumber .
The world around us is awash in waves, from the visible light that allows us to see, to the invisible radio waves that carry our communications. Characterizing these waves accurately is crucial in countless scientific and technological applications. Two fundamental properties used to describe waves are wavelength and wavenumber. Understanding their relationship is essential for anyone working with electromagnetic radiation, particularly in fields like spectroscopy.
This guide aims to provide a comprehensive understanding of the wavelength to wavenumber conversion. We will delve into the definitions, units, and mathematical relationships between these two properties. By the end of this guide, you will have a solid foundation for accurately converting between wavelength and wavenumber, and understanding the significance of this conversion.
Defining Wavelength and Wavenumber
Wavelength (represented by the Greek letter lambda, λ) is the distance between two successive crests or troughs of a wave. It's a measure of the spatial period of the wave. The standard unit of wavelength is the meter (m), but it is also commonly expressed in centimeters (cm) or nanometers (nm), especially when dealing with light.
Wavenumber (represented by the Greek letter nu, ν, or sometimes k) is the spatial frequency of a wave, defined as the number of wavelengths per unit distance. It is essentially the reciprocal of the wavelength. The standard unit of wavenumber is inverse centimeters (cm-1), although inverse meters (m-1) are also used.
Purpose of This Guide
This guide's primary purpose is to equip you with the knowledge and tools necessary to confidently and accurately convert between wavelength and wavenumber. We will break down the core equation that links these two properties, and provide a step-by-step conversion process.
Whether you are a student learning about spectroscopy, a researcher analyzing spectral data, or a professional working with electromagnetic radiation, this guide will serve as a valuable resource.
Applications in Spectroscopy and Beyond
The relationship between wavelength and wavenumber is particularly crucial in spectroscopy. Spectroscopic techniques analyze the interaction of electromagnetic radiation with matter. By measuring the wavelengths or wavenumbers of absorbed or emitted radiation, scientists can identify the composition of a substance and determine its properties.
For example, Infrared (IR) spectroscopy uses wavenumber to analyze the vibrational modes of molecules, providing a "fingerprint" that can identify specific compounds. Raman spectroscopy, another powerful technique, also relies heavily on wavenumber to analyze scattered light and gain insights into molecular structure and dynamics.
Beyond spectroscopy, the understanding of wavelength and wavenumber finds applications in various other fields, including:
-
Telecommunications: Characterizing the frequencies of radio waves.
-
Materials Science: Analyzing the optical properties of materials.
-
Astronomy: Studying the light emitted by distant stars and galaxies.
The ability to convert between wavelength and wavenumber is a fundamental skill that unlocks a deeper understanding of the physical world.
The previous section introduced the fundamental concepts of wavelength and wavenumber, establishing their importance in fields like spectroscopy. Now, let's delve deeper into these essential wave properties. We'll explore their precise definitions, the units we use to measure them, and their physical significance. A solid grasp of these foundational elements is key to confidently converting between wavelength and wavenumber and applying these concepts effectively.
Deciphering Wavelength and Wavenumber: Definitions and Units
Understanding Wavelength (λ)
Wavelength, denoted by the Greek letter lambda (λ), is a fundamental property of waves that describes the spatial distance over which the wave's shape repeats. More specifically, it is the distance between two successive crests or troughs of a wave. Imagine a ripple in a pond; the wavelength is the distance from one peak of a ripple to the next.
Understanding wavelength is crucial because it dictates how a wave interacts with matter. Shorter wavelengths, like those of ultraviolet light or X-rays, carry more energy and can be more damaging to biological tissues. Longer wavelengths, like those of radio waves, are used for communication and broadcasting.
Units of Measurement for Wavelength
Wavelength is a measure of distance, and its standard unit in the International System of Units (SI) is the meter (m). However, depending on the type of wave being studied, other units are often more convenient.
- Nanometers (nm): One nanometer is equal to one billionth of a meter (1 nm = 10-9 m). Nanometers are commonly used to express the wavelengths of visible light and ultraviolet radiation.
- Centimeters (cm): One centimeter is equal to one hundredth of a meter (1 cm = 10-2 m). Centimeters are often used when describing infrared radiation or microwaves.
The choice of unit depends on the scale of the wavelengths being considered. Using appropriate units makes the values more manageable and easier to interpret.
Wavelength and the Electromagnetic Spectrum
The electromagnetic spectrum is the range of all possible electromagnetic radiation frequencies. This spectrum spans from extremely long radio waves to very short gamma rays. Wavelength plays a key role in defining the position of a wave on this spectrum.
Different regions of the electromagnetic spectrum are characterized by different ranges of wavelengths. For example:
- Radio waves: Have wavelengths ranging from millimeters to hundreds of meters.
- Microwaves: Have wavelengths ranging from approximately 1 mm to 1 meter.
- Infrared radiation: Has wavelengths ranging from about 700 nm to 1 mm.
- Visible light: Has wavelengths ranging from approximately 400 nm (violet) to 700 nm (red).
- Ultraviolet radiation: Has wavelengths ranging from about 10 nm to 400 nm.
- X-rays: Have wavelengths ranging from approximately 0.01 nm to 10 nm.
- Gamma rays: Have wavelengths shorter than approximately 0.01 nm.
The wavelength of electromagnetic radiation dictates its interaction with matter. This interaction forms the basis for a vast range of technologies and scientific investigations.
Understanding Wavenumber (ν or k)
Wavenumber, denoted by the Greek letter nu (ν) or sometimes k, represents the spatial frequency of a wave. Unlike wavelength, which measures the distance of one complete wave cycle, wavenumber quantifies the number of wave cycles that exist per unit distance.
Think of it this way: If you have a string stretched across a certain length, the wavenumber tells you how many full "waves" fit along that string.
Units of Measurement for Wavenumber
The standard unit of wavenumber is inverse centimeters (cm-1), although inverse meters (m-1) are also used, albeit less commonly. This unit reflects the definition of wavenumber as the number of waves per centimeter (or meter).
It’s important to note that the units of wavenumber are reciprocal units. This is because wavenumber is inversely proportional to wavelength. A higher wavenumber indicates a shorter wavelength and vice-versa.
Physical Significance of Wavenumber
Wavenumber is particularly useful in spectroscopy and related fields because it is directly proportional to the energy of a photon.
This relationship is expressed by the equation: E = hcν, where:
- E is the energy of the photon
- h is Planck's constant
- c is the speed of light
- ν is the wavenumber
This equation highlights that radiation with a higher wavenumber possesses greater energy.
In molecular spectroscopy, wavenumber is used to characterize the vibrational and rotational modes of molecules. When a molecule absorbs infrared radiation, it transitions to a higher vibrational energy level. The specific wavenumbers at which absorption occurs provide information about the molecule's structure and composition.
For example, in infrared (IR) spectroscopy, the positions of absorption bands (expressed in cm-1) are used to identify functional groups within a molecule, providing a fingerprint for its identification. The vibrations of chemical bonds (like C=O, O-H, or C-H) each absorb infrared light at characteristic wavenumbers.
By analyzing the infrared spectrum of a substance, scientists can identify the chemical compounds present and gain insights into the molecular structure. In essence, wavenumber serves as a direct link between the observed spectral data and the underlying molecular properties.
The interplay between wavelength and wavenumber is a cornerstone of spectroscopic analysis. Understanding their mathematical relationship is essential for accurately interpreting spectral data and extracting meaningful information about the molecular world.
The Core Equation: Unveiling the Mathematical Link
The mathematical bridge connecting wavelength and wavenumber is elegantly simple yet profoundly powerful. This relationship reveals the inverse nature of these two fundamental wave properties and allows for seamless conversion between them.
The Fundamental Formula: ν = 1/λ
At its heart, the relationship between wavenumber (ν) and wavelength (λ) is defined by the formula:
ν = 1/λ
This equation states that wavenumber is simply the reciprocal of wavelength. This inverse relationship is critical to understand. As wavelength increases, wavenumber decreases, and vice versa.
Dissecting the Inverse Relationship
The inverse relationship between wavelength and wavenumber means they provide complementary ways of describing a wave. A long wavelength corresponds to a low wavenumber, indicating lower energy.
Conversely, a short wavelength corresponds to a high wavenumber, indicating higher energy. This is crucial in spectroscopy, where energy levels are directly related to molecular transitions.
It's also worth noting that some texts use k to denote wavenumber, especially in solid-state physics. Therefore, k = 1/λ may also be encountered.
The Mathematics of Conversion and Unit Consistency
The formula ν = 1/λ is straightforward, but applying it correctly requires careful attention to units. If wavelength is measured in centimeters (cm), the resulting wavenumber will be in inverse centimeters (cm-1), a common unit in spectroscopy.
If wavelength is in meters (m), the wavenumber will be in inverse meters (m-1). The consistency of units is paramount. Failure to maintain consistency will lead to incorrect conversions and erroneous results.
For example, if you have a wavelength of 500 nm, you must first convert it to cm (500 nm = 5 x 10-5 cm) before calculating the wavenumber:
ν = 1 / (5 x 10-5 cm) = 20,000 cm-1
Always double-check your units before, during, and after the calculation.
The Interplay of Speed of Light (c) and Frequency (f)
Wavelength and wavenumber are not the only properties that describe a wave. Frequency (f) and the speed of light (c) are also inextricably linked. These parameters provide a more complete picture of wave behavior.
These values are also critical for understanding the energy associated with electromagnetic radiation.
Connecting Wavelength, Frequency, and the Speed of Light
The speed of light (c), frequency (f), and wavelength (λ) are related by the equation:
c = λf
This equation highlights that the speed of light is constant. Therefore, wavelength and frequency are inversely proportional.
Wavenumber can be expressed in terms of frequency and the speed of light:
ν = f/c
This equation connects wavenumber directly to frequency, emphasizing that wavenumber is proportional to frequency. Higher frequency radiation has a higher wavenumber. This relationship becomes particularly important when considering the energy of photons.
The previous section established the fundamental mathematical relationship between wavelength and wavenumber. Now, let's translate that theoretical understanding into a practical skill. The following steps will guide you through the conversion process, ensuring accurate and reliable results.
Step-by-Step Conversion: A Practical Guide
This section focuses on the practical application of the wavenumber-wavelength relationship. It provides a clear, step-by-step guide to converting between these two units, complete with examples and cautionary notes.
Converting Wavelength to Wavenumber: A Detailed Process
The process of converting wavelength to wavenumber is fundamentally about applying the reciprocal relationship. However, attention to detail, especially regarding units, is paramount.
Here's a breakdown of the steps:
-
Identify the Wavelength Value and its Unit: The first crucial step is to accurately identify the wavelength value you are starting with and its corresponding unit of measurement. Wavelength is commonly expressed in units like nanometers (nm), micrometers (µm), centimeters (cm), or meters (m).
-
Ensure Consistent Units: The key to accurate conversion lies in unit consistency. The wavenumber is almost universally expressed in inverse centimeters (cm-1). Therefore, if your wavelength is in any other unit, it must be converted to centimeters before applying the formula.
-
Apply the Conversion Formula: Once the wavelength is expressed in centimeters, the conversion is straightforward:
ν = 1 / λ
Where: ν is the wavenumber (in cm-1) λ is the wavelength (in cm)
-
Calculate the Wavenumber: Perform the division to obtain the wavenumber. The result will be in cm-1.
-
Report the Result with Correct Significant Figures: Pay attention to significant figures. The final wavenumber should reflect the precision of your initial wavelength measurement.
Example Calculations: Mastering the Conversion
Let's illustrate the conversion process with a few examples, covering different wavelength units:
Example 1: Nanometers (nm) to cm-1
Convert a wavelength of 500 nm to wavenumber.
- Wavelength: λ = 500 nm
- Convert nm to cm: 1 nm = 1 x 10-7 cm, therefore 500 nm = 500 x 10-7 cm = 5 x 10-5 cm
- Apply the formula: ν = 1 / (5 x 10-5 cm)
- Calculate: ν = 20,000 cm-1
Example 2: Micrometers (µm) to cm-1
Convert a wavelength of 2.5 µm to wavenumber.
- Wavelength: λ = 2.5 µm
- Convert µm to cm: 1 µm = 1 x 10-4 cm, therefore 2.5 µm = 2.5 x 10-4 cm
- Apply the formula: ν = 1 / (2.5 x 10-4 cm)
- Calculate: ν = 4,000 cm-1
Example 3: Meters (m) to cm-1
Convert a wavelength of 1.0 x 10-6 m to wavenumber.
- Wavelength: λ = 1.0 x 10-6 m
- Convert m to cm: 1 m = 100 cm, therefore 1.0 x 10-6 m = 1.0 x 10-4 cm
- Apply the formula: ν = 1 / (1.0 x 10-4 cm)
- Calculate: ν = 10,000 cm-1
Common Pitfalls and How to Avoid Them
While the conversion formula is simple, errors can arise from a few common mistakes:
-
Unit Inconsistencies: This is the most frequent source of error. Always ensure wavelength is in centimeters before calculating the wavenumber. Use conversion factors diligently.
-
Incorrect Scientific Notation: Pay close attention to the exponents in scientific notation, especially when dealing with very small or very large numbers.
-
Significant Figures: Maintain the appropriate number of significant figures throughout the calculation and in the final result. The precision of the wavenumber cannot exceed the precision of the original wavelength measurement.
-
Calculator Errors: Double-check your entries in calculators, especially when using scientific notation or complex expressions. It's helpful to estimate the expected result to catch gross errors.
Emphasizing Unit Consistency and Conversion Tables
As highlighted earlier, consistent units are crucial. A conversion table can be a valuable tool for quickly converting between different units of length:
| Unit | Conversion to cm |
|---|---|
| Meter (m) | 100 cm |
| Centimeter (cm) | 1 cm |
| Millimeter (mm) | 0.1 cm |
| Micrometer (µm) | 1 x 10-4 cm |
| Nanometer (nm) | 1 x 10-7 cm |
| Angstrom (Å) | 1 x 10-8 cm |
By following these steps, paying attention to detail, and utilizing the provided examples and conversion table, you can confidently and accurately convert between wavelength and wavenumber.
The previous section established the fundamental mathematical relationship between wavelength and wavenumber. Now, let's translate that theoretical understanding into a practical skill. The following steps will guide you through the conversion process, ensuring accurate and reliable results.
Applications in Spectroscopy: Unlocking Molecular Secrets
Spectroscopy, a cornerstone of modern analytical chemistry and materials science, relies heavily on the concept of wavenumber. Wavenumber provides a direct link to the energy levels of molecules, allowing scientists to probe their structure, composition, and dynamics. This section explores the crucial role of wavenumber in various spectroscopic techniques, showcasing its power in unlocking molecular secrets.
The Spectroscopic Significance of Wavenumber
Wavenumber isn't merely an alternative unit to wavelength; it holds intrinsic physical significance. In spectroscopy, wavenumber is directly proportional to the energy of the absorbed or emitted radiation. This direct relationship simplifies data interpretation, especially when analyzing complex spectra. It allows researchers to connect spectral features directly to specific molecular vibrations or electronic transitions.
Infrared (IR) Spectroscopy: Vibrational Fingerprints
IR spectroscopy is a widely used technique that exploits the vibrational modes of molecules. When a molecule absorbs infrared radiation, it undergoes vibrational excitation.
The specific wavenumbers at which absorption occurs are determined by the molecule's structure and the nature of its chemical bonds. Each molecule possesses a unique IR spectrum, often referred to as its "vibrational fingerprint."
Functional Group Identification
IR spectroscopy excels at identifying functional groups within a molecule. Specific functional groups, such as carbonyls (C=O), hydroxyls (O-H), and amines (N-H), absorb IR radiation at characteristic wavenumbers. By analyzing the IR spectrum, chemists can readily identify the presence or absence of these groups, providing valuable insights into the molecule's structure.
Analyzing Molecular Vibrations
The precise position and intensity of IR bands provide information about the strength and nature of chemical bonds. Shifts in wavenumber can indicate changes in bond order or the presence of intermolecular interactions. This makes IR spectroscopy a powerful tool for studying molecular dynamics and chemical reactions.
Raman Spectroscopy: Complementary Insights
Raman spectroscopy provides complementary information to IR spectroscopy. It is based on the Raman effect, which involves the inelastic scattering of light by molecules.
While IR spectroscopy measures absorption, Raman spectroscopy measures scattering. The change in wavenumber between the incident and scattered light corresponds to the vibrational energy of the molecule.
Advantages of Raman Spectroscopy
Raman spectroscopy offers several advantages over IR spectroscopy. It is less sensitive to water, making it suitable for analyzing aqueous samples. It can also be used to study non-polar molecules, which are often IR-inactive. Furthermore, Raman spectroscopy is particularly sensitive to vibrations involving symmetric bonds.
Structural Elucidation
By combining IR and Raman spectroscopy, scientists can obtain a more complete picture of a molecule's vibrational modes. This combined approach provides valuable information for structural elucidation, allowing researchers to determine the arrangement of atoms within a molecule.
Identifying Substances and Analyzing Properties Using Spectral Data
Spectroscopic data, with wavenumber as the key parameter, provides a robust and reliable method for identifying substances. Spectral databases containing reference spectra of numerous compounds allow for comparison and matching.
Spectral Fingerprinting
The unique spectral fingerprint of a compound, whether obtained through IR, Raman, or other spectroscopic techniques, serves as a definitive identifier. By comparing an unknown spectrum to a database of known spectra, researchers can quickly and accurately identify the substance.
Quantitative Analysis
Furthermore, wavenumber-resolved spectral data enables quantitative analysis. The intensity of absorption or scattering at specific wavenumbers is directly proportional to the concentration of the substance. This relationship allows for the determination of the amount of a particular compound in a sample.
Beyond Traditional Spectroscopy: Other Applications
While wavenumber finds its primary application in vibrational spectroscopy, its utility extends beyond these realms. It is used in various forms of optical spectroscopy, including UV-Vis spectroscopy for analyzing electronic transitions, and in characterizing optical materials and devices.
Wavenumber, therefore, serves as a unifying concept across diverse spectroscopic and analytical techniques, making it an indispensable tool for scientists across numerous disciplines.
The power of wavenumber in spectroscopic analysis is undeniable, allowing for direct correlation between spectral data and molecular properties. To further streamline this process, a variety of tools and resources have emerged, offering quick and efficient wavelength to wavenumber conversions. This section provides an overview of these resources, offering guidance on selecting the most appropriate tool and highlighting the importance of verifying the results.
Tools and Resources: Simplifying the Conversion Process
The digital landscape is populated with numerous online conversion tools and calculators designed to facilitate wavelength to wavenumber conversions. These tools range from simple, single-purpose calculators to more comprehensive spectral analysis platforms. Understanding the features, limitations, and appropriate use of these resources is crucial for accurate and reliable results.
Navigating the Online Conversion Landscape
Online conversion tools offer an immediate solution for converting between wavelength and wavenumber. Many websites provide free, readily accessible calculators. These tools typically require the user to input the wavelength value and select the appropriate units, after which the wavenumber is automatically calculated and displayed.
However, the ease of access should not come at the expense of accuracy. It’s important to understand the tool’s underlying algorithms and ensure that it handles unit conversions correctly.
Choosing the Right Tool: Accuracy and Ease of Use
Selecting the optimal conversion tool depends on several factors, including the required accuracy, the complexity of the calculation, and the user's technical expertise. Here are some factors to consider:
- Accuracy: Prioritize tools that provide results with sufficient precision for your specific application. Some tools may offer a limited number of significant figures, which can be problematic when dealing with high-resolution spectral data.
- Unit Support: Ensure the tool supports the units relevant to your data (e.g., nanometers, micrometers, centimeters). The ability to seamlessly convert between different units can save time and reduce the risk of errors.
- User Interface: A clean and intuitive user interface is essential for efficient use. Look for tools that are easy to navigate and provide clear instructions.
- Additional Features: Some advanced tools offer additional features, such as the ability to perform spectral simulations or analyze data from multiple sources. Consider whether these features are relevant to your needs.
The Importance of Verification: Mitigating Potential Errors
Regardless of the chosen tool, verifying the results is an essential step in the conversion process. Online calculators, while convenient, are not infallible. Errors can arise from incorrect unit selection, software glitches, or limitations in the tool's algorithms.
To mitigate these risks, it is recommended to:
- Cross-check with Multiple Tools: Compare the results obtained from different online calculators. If significant discrepancies exist, investigate the source of the error.
- Manual Calculation: Perform a manual calculation using the fundamental formula (ν = 1/λ) to verify the results independently. This step can help identify errors in unit conversions or other calculation mistakes.
- Unit Analysis: Always double-check the units of your input and output values. Ensure that the tool correctly handles unit conversions and that the final result is expressed in the desired units.
By adopting a critical and rigorous approach to using online conversion tools, researchers can ensure the accuracy and reliability of their wavelength to wavenumber conversions. This ultimately leads to more meaningful and insightful spectroscopic analysis.
The ability to seamlessly convert between wavelength and wavenumber provides immediate benefits for spectral data analysis. However, those interested in delving deeper into the fundamental physics at play can leverage wavenumber to unlock an even greater understanding of energy at the molecular level.
Advanced Concepts: Unveiling the Energy-Wavenumber Relationship
For those seeking a more profound understanding of spectroscopic principles, exploring the connection between wavenumber and energy opens a gateway to the quantum world. This relationship, governed by Planck's constant, provides a powerful framework for interpreting spectral data in terms of energy transitions within molecules.
The Quantum Connection: Planck's Constant and Energy
At the heart of this connection lies Planck's constant (h), a fundamental constant in quantum mechanics. It quantifies the relationship between energy and frequency of electromagnetic radiation. The energy (E) of a photon is directly proportional to its frequency (ν), as described by the equation E = hν.
Since wavenumber (ν̃) is related to frequency (ν) by the speed of light (c), ν = cν̃, we can rewrite the energy equation as E = hcν̃. This equation reveals a direct proportionality between energy and wavenumber.
This relationship is profound: it establishes that the wavenumber of a spectral line is directly proportional to the energy change associated with a molecular transition.
Interpreting Spectral Data through Energy Levels
This energy-wavenumber relationship is crucial for interpreting spectral data in terms of molecular energy levels. When a molecule absorbs a photon, it transitions to a higher energy state. The wavenumber of the absorbed photon corresponds to the energy difference between the initial and final states.
By analyzing the wavenumbers of absorption or emission lines in a spectrum, we can gain insights into the quantized energy levels of a molecule. This information is invaluable for understanding molecular structure, dynamics, and reactivity.
Implications for Quantum Mechanics
The energy-wavenumber relationship has significant implications for quantum mechanics. It demonstrates the quantization of energy, a cornerstone of quantum theory. Molecular energy levels are not continuous but rather discrete, meaning that molecules can only exist in specific energy states.
Spectroscopy, through the lens of wavenumber and its connection to energy, provides experimental evidence for the principles of quantum mechanics. It allows us to probe the quantum nature of matter at the molecular level, revealing the intricate interplay between energy, frequency, and molecular properties.
A Deeper Dive into Molecular Behavior
By embracing these advanced concepts, researchers can move beyond simply identifying substances using spectral data. They can gain a deeper understanding of the underlying physical processes that govern molecular behavior. The energy-wavenumber relationship provides a powerful tool for unraveling the secrets of the quantum world and exploring the fundamental nature of matter.
Video: Wavelength to Wavenumber: The Complete Conversion Guide
Wavelength to Wavenumber Conversion: Frequently Asked Questions
Here are some common questions about converting wavelength to wavenumber, a vital process in various scientific fields.
What exactly is wavenumber and how does it relate to wavelength?
Wavenumber represents the number of waves per unit distance, typically per centimeter (cm⁻¹). It's the spatial frequency of a wave. Wavelength, conversely, is the distance between identical points (e.g., crests or troughs) on adjacent waves. Wavenumber is inversely proportional to wavelength.
Why would I need to convert wavelength to wavenumber?
Scientists frequently use wavenumber, especially in spectroscopy, because it is directly proportional to energy. This makes it easier to relate spectral data to molecular vibrations and energy levels. Converting wavelength to wavenumber is a common calculation to obtain this more useful energy-related value.
What's the formula for converting wavelength to wavenumber?
The conversion formula is simple: Wavenumber (in cm⁻¹) = 1 / Wavelength (in cm). If your wavelength is in meters (m), you can convert it to centimeters first (1 m = 100 cm), or use: Wavenumber (in cm⁻¹) = 100 / Wavelength (in m).
Is converting wavelength to wavenumber always necessary?
No, it isn't always necessary. The choice depends on the specific application and what information is most relevant. However, if you need to relate your data to energy or analyze spectral properties, converting from wavelength to wavenumber will usually provide more direct insights.