Unlock Kinetic Energy: Simple Guide Everyone Needs!
Understanding thermodynamics is crucial for grasping the principles behind energy transfer and transformation. Boltzmann constant represents the relationship between temperature and energy at the molecular level, fundamentally influencing average kinetic energy in a system or object. Specifically, the Massachusetts Institute of Technology (MIT) constantly pushes the boundaries of physics research to deepen our understanding of molecular dynamics and its connection to energy. Average kinetic energy within a system or object greatly influences these relationships, which is fundamental for predicting material behavior.

Image taken from the YouTube channel Professor Dave Explains , from the video titled Kinetic Energy and Potential Energy .
Unlocking the Secrets of Kinetic Energy
Kinetic energy, the energy of motion, is a fundamental concept permeating our everyday experiences. From a leisurely stroll to a speeding car, every moving object possesses kinetic energy. Understanding this energy unlocks insights into the behavior of physical systems, from the smallest molecules to the largest celestial bodies.
Kinetic Energy: The Essence of Movement
At its core, kinetic energy quantifies the energy an object possesses due to its motion. Any object in motion—whether it be a thrown baseball, a flowing river, or even air molecules vibrating in place—exhibits kinetic energy. The faster it moves and the more mass it has, the greater its kinetic energy.
Kinetic energy plays a crucial role in numerous physical processes. It is essential for understanding collisions, energy transfer, and the behavior of fluids and gases. In essence, kinetic energy is a building block for comprehending how the world around us functions.
The Significance of Average Kinetic Energy
While understanding basic kinetic energy is important, grasping the concept of average kinetic energy is even more vital, especially when dealing with systems containing many particles. Imagine a container of gas: the individual molecules are zipping around at varying speeds, each possessing its own kinetic energy.
Instead of tracking the kinetic energy of each molecule (an impossible task), we focus on the average. This "average kinetic energy" provides a representative measure of the system's overall energy state, offering insights into temperature, pressure, and other macroscopic properties.
A Guide to Understanding
This article aims to demystify the concept of average kinetic energy, presenting a clear and accessible guide to its definition, applications, and importance. We will explore the relationship between average kinetic energy and temperature, delve into the relevant formulas, and showcase real-world examples.
Whether you are a student, a science enthusiast, or simply curious about the world around you, this guide will equip you with a solid understanding of average kinetic energy. Get ready to unlock the secrets of motion.
That exploration of average kinetic energy naturally leads us to a deeper understanding of kinetic energy itself, the foundation upon which the concept of averages is built. So, before we delve further into the intricacies of average kinetic energy, let's solidify our grasp of the fundamental principles that govern the energy of motion.
Kinetic Energy: The Basics
Kinetic energy, at its heart, is the energy possessed by an object due to its motion.
It's not a hidden or latent form of energy; it's directly observable and quantifiable. If something is moving, it has kinetic energy.
Defining Kinetic Energy
More formally, kinetic energy is the measure of the work an object can do by virtue of its movement. This definition connects it to the broader concept of energy as the ability to do work.
A stationary object has no kinetic energy, but the moment it begins to move, it gains kinetic energy proportional to its mass and speed.
The Kinetic Energy Formula
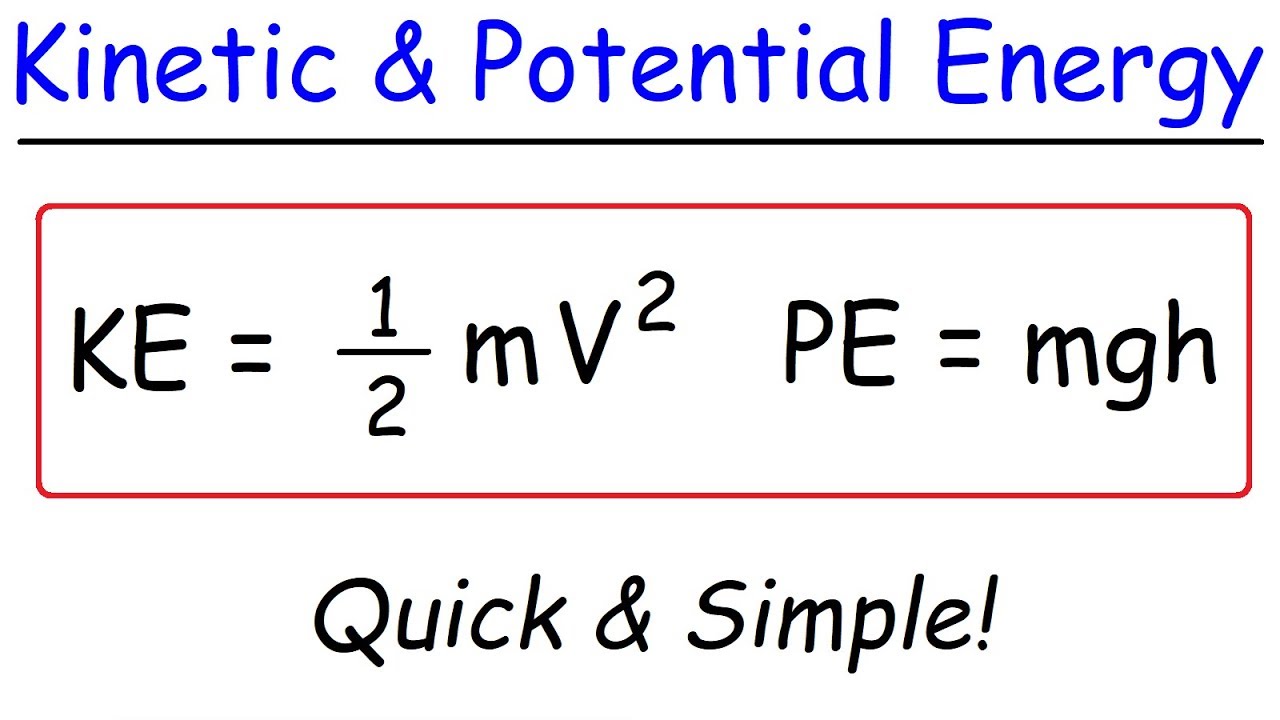
The relationship between kinetic energy (KE), mass (m), and velocity (v) is elegantly expressed by the following formula:
KE = 1/2 m v2
This equation is the cornerstone of understanding kinetic energy. It reveals the precise mathematical connection between an object's properties and its energy of motion.
The Impact of Mass and Velocity
The kinetic energy formula reveals that both mass and velocity influence an object's kinetic energy, but their impact differs significantly.
The Role of Mass
Mass is directly proportional to kinetic energy. If you double the mass of an object while keeping its velocity constant, you double its kinetic energy.
A heavier object moving at the same speed as a lighter one will always possess more kinetic energy.
The Dominance of Velocity
Velocity, however, plays a more dominant role. Because velocity is squared in the formula, its impact on kinetic energy is exponential.
If you double the velocity of an object while keeping its mass constant, you quadruple its kinetic energy.
This is why even small increases in speed can lead to substantial increases in kinetic energy. For example, the destructive power of a speeding bullet, despite its small mass, is a direct consequence of its extremely high velocity.
Therefore, understanding the individual contributions of mass and, especially, velocity is key to comprehending the magnitude of kinetic energy in any moving object.
That exploration of average kinetic energy naturally leads us to a deeper understanding of kinetic energy itself, the foundation upon which the concept of averages is built. So, before we delve further into the intricacies of average kinetic energy, let's solidify our grasp of the fundamental principles that govern the energy of motion.
Why "Average" Kinetic Energy Matters
The term "average" is more than just a mathematical convenience; it's essential when we're dealing with systems composed of countless particles. In the microscopic world, uniformity is a myth. Instead, we encounter a bustling arena of constant motion, with each particle possessing its own unique velocity.
The Reality of Particle Systems
Imagine a container of gas. It's not a static entity but a dynamic collection of molecules zipping around at varying speeds. Some might be sluggishly meandering, while others are hurtling with considerable momentum. Each molecule, therefore, possesses its own individual kinetic energy.
In systems like these, asking about the kinetic energy is inherently flawed. There isn't a single value that accurately represents the system. We are dealing with a distribution of kinetic energies, a spectrum of motion.
The Need for a Representative Value
If we can't pinpoint a single kinetic energy, how do we characterize the system's overall state? This is where the concept of "average" becomes indispensable. We need a single, representative value to describe the typical kinetic energy within the system.
That single, representative value that we focus on is the mean kinetic energy value.
Focusing on the Mean
The average kinetic energy provides a concise and useful way to describe the overall energy state of the system. Instead of tracking each particle's energy individually (an impossible task for macroscopic systems), we focus on the mean kinetic energy.
This "average" allows us to relate the microscopic world of particle motion to macroscopic properties like temperature and pressure, giving us a powerful tool for understanding and predicting the behavior of complex systems.
That single, representative value that we focus on is the mean kinetic energy value. This concept of a representative value naturally segues into another fundamental question: What dictates this average kinetic energy? The answer lies in the intrinsic link between kinetic energy and temperature.
Temperature and Kinetic Energy: A Direct Relationship
Temperature, often perceived as a measure of hotness or coldness, is fundamentally tied to the microscopic world of particle motion. It serves as a macroscopic indicator of the average kinetic energy possessed by the particles within a system.
Unveiling the Connection
The higher the temperature of a substance, the more vigorously its constituent particles move. This increased motion translates directly into higher kinetic energy. Conversely, lower temperatures imply slower particle movement and, consequently, lower kinetic energy.
This relationship isn't merely coincidental; it's a fundamental principle governing the behavior of matter.
It's vital to understand that temperature doesn't measure the total kinetic energy, but rather the average kinetic energy of the particles.
The Dance of Molecules: Temperature's Influence
Imagine heating a container of gas. As the temperature rises, the gas molecules absorb energy. This energy fuels their motion, causing them to move faster and collide more frequently with each other and the container walls.
This increased molecular agitation is the direct manifestation of heightened kinetic energy. In essence, temperature is a proxy for the intensity of this molecular dance.
Introducing the Boltzmann Constant: The Bridge
While the link between temperature and average kinetic energy is clear, quantifying this relationship requires a crucial constant: the Boltzmann constant, denoted as k.
The Boltzmann constant acts as a bridge, connecting the macroscopic world of temperature, measured in Kelvin, to the microscopic realm of particle energy, measured in Joules.
Its value, approximately 1.38 x 10-23 Joules per Kelvin, provides the scaling factor necessary to translate temperature into average kinetic energy.
The significance of the Boltzmann constant lies in its universality. It's a fundamental constant of nature, applicable to a wide range of systems, from ideal gases to complex biological systems.
The Boltzmann constant is the lynchpin that connects temperature and the average kinetic energy of particles within a system. This is not just theoretical; it is the basis for many practical applications.
That link between temperature and average kinetic energy is clear, quantifying this relationship requires a specific formula. This formula serves as a powerful tool for calculating the average kinetic energy of particles within a system, given its temperature.
The Average Kinetic Energy Formula Explained
The average kinetic energy isn't some abstract concept; it's a quantifiable value, meticulously defined by a concise equation. This formula unlocks deeper insights into the behavior of matter at a microscopic level.
The formula for average kinetic energy is expressed as:
KEavg = (3/2) k T
Where:
- KEavg represents the average kinetic energy of a particle in the system.
- k is the Boltzmann constant, approximately 1.38 x 10-23 J/K (Joules per Kelvin).
- T is the absolute temperature of the system, measured in Kelvin.
Decoding the "3/2" Factor: Degrees of Freedom
The seemingly arbitrary "3/2" factor in the formula arises from the concept of degrees of freedom.
In three-dimensional space, a particle can move independently along three axes: x, y, and z. Each of these axes represents a degree of freedom for translational motion.
The equipartition theorem dictates that, at thermal equilibrium, each degree of freedom contributes (1/2) k T to the average energy of the particle. Since we have three degrees of freedom, the total contribution becomes (3/2) k T.
It's important to note that this "3/2" factor applies specifically to monatomic ideal gases, where energy is primarily translational.
For more complex molecules with rotational and vibrational degrees of freedom, the factor will be different, reflecting the additional ways the molecule can store energy.
Root Mean Square Velocity (Vrms): Kinetic Energy's Partner
Another critical concept closely linked to average kinetic energy is the Root Mean Square Velocity (Vrms).
Vrms provides a measure of the "typical" speed of particles in a system. It's not simply the average of the velocities, but rather the square root of the average of the squared velocities. This subtle difference accounts for the distribution of speeds and ensures a more accurate representation of the system's kinetic state.
The Vrms is related to the average kinetic energy as follows:
KEavg = (1/2) m Vrms2
Where m represents the mass of a single particle.
Rearranging this formula, we can express Vrms in terms of temperature:
Vrms = √((3 k T) / m)
This equation highlights a crucial relationship: at a given temperature, lighter particles will have a higher Vrms than heavier particles. This explains why lighter gases diffuse faster than heavier ones.
That link between temperature and average kinetic energy is clear, quantifying this relationship requires a specific formula. This formula serves as a powerful tool for calculating the average kinetic energy of particles within a system, given its temperature. Now, let's explore how this microscopic understanding of kinetic energy connects to macroscopic properties like pressure and volume, particularly within the realm of gases. This connection is beautifully illustrated by the Ideal Gas Law.
Kinetic Energy and the Ideal Gas Law
The Ideal Gas Law is a cornerstone of thermodynamics.
It offers a simplified yet powerful model to describe the behavior of gases under specific conditions.
It elegantly combines pressure, volume, temperature, and the number of moles of a gas into a single equation:
PV = nRT
Where:
- P represents the pressure of the gas.
- V is the volume occupied by the gas.
- n signifies the number of moles of gas.
- R is the ideal gas constant (approximately 8.314 J/(mol·K)).
- T is the absolute temperature in Kelvin.
Deriving the Ideal Gas Law from Kinetic Theory
What's fascinating is that the Ideal Gas Law isn't just an empirical observation; it's deeply rooted in the molecular kinetic theory of gases.
This theory postulates that gases are composed of a large number of particles in constant, random motion.
These particles are assumed to have negligible volume themselves and experience no intermolecular forces except during collisions.
Through statistical mechanics and considering the average kinetic energy of these particles, we can arrive at the Ideal Gas Law.
The derivation hinges on the understanding that the pressure exerted by a gas on the walls of its container is a direct result of the countless collisions of gas molecules with those walls.
The force of each collision depends on the mass and velocity of the molecule.
The frequency of collisions depends on the number of molecules and their average speed.
Linking these microscopic properties (mass, velocity, number of molecules) to the macroscopic properties of pressure and volume reveals the fundamental relationship expressed in PV = nRT.
The Interplay of Kinetic Energy, Pressure, and Volume
The Ideal Gas Law beautifully demonstrates how kinetic energy, pressure, and volume are intertwined.
Consider a fixed volume of gas (V is constant). If we increase the temperature (T), the average kinetic energy of the gas molecules increases.
This means they move faster and collide more forcefully and frequently with the container walls.
Consequently, the pressure (P) increases proportionally.
Conversely, if we increase the volume (V) while keeping the temperature (T) constant, the gas molecules have more space to move around in.
This reduces the frequency of collisions with the walls, leading to a decrease in pressure (P).
In essence, the Ideal Gas Law provides a macroscopic window into the microscopic world of molecular motion.
It directly relates the observable properties of pressure, volume, and temperature to the average kinetic energy of the gas molecules.
Kinetic Energy Across Different States of Matter
Having established the fundamental link between kinetic energy and the behavior of gases, it's natural to wonder how this energy manifests itself across the three fundamental states of matter: solid, liquid, and gas. While the average kinetic energy is intrinsically linked to temperature in all states, the manifestation and influence of this energy differ considerably due to variations in intermolecular forces and particle arrangements.
Kinetic Energy in Solids: Vibration and Constraint
In solids, particles (atoms, ions, or molecules) are tightly packed in a fixed lattice structure. They are not free to move translationally as they are in liquids and gases. Instead, their kinetic energy is primarily expressed as vibrational energy around their equilibrium positions.
The strength of the intermolecular forces in a solid dictates the amplitude and frequency of these vibrations. Stronger forces result in lower vibrational amplitudes, while weaker forces allow for more extensive movement.
Increasing the temperature of a solid causes the particles to vibrate more vigorously, increasing their average kinetic energy and, consequently, the overall internal energy of the solid. If enough thermal energy is supplied, the vibrations can overcome the intermolecular forces, leading to a phase transition from solid to liquid (melting).
Kinetic Energy in Liquids: Flow and Intermolecular Play
Liquids occupy an intermediate position between solids and gases in terms of kinetic energy and intermolecular forces. Particles in a liquid are still in close proximity to one another, but they possess sufficient kinetic energy to overcome the fixed arrangement characteristic of solids.
This allows them to move translationally, rotationally, and vibrationally, although with more constraints than gases. The average kinetic energy of liquid particles is reflected in their ability to flow and conform to the shape of their container.
Intermolecular forces in liquids play a crucial role in governing their properties. These forces, such as Van der Waals forces, hydrogen bonding, and dipole-dipole interactions, influence the viscosity, surface tension, and boiling point of the liquid.
The stronger these intermolecular forces, the more energy is required to overcome them and allow the liquid to transition into a gaseous state.
Kinetic Energy in Gases: Freedom and Temperature Dependence
In the gaseous state, particles are widely dispersed and exhibit minimal intermolecular interactions under ideal conditions. Their kinetic energy is primarily manifested as translational motion, with particles moving randomly and independently through space.
The average kinetic energy of gas particles is directly proportional to the absolute temperature of the gas, as described by the kinetic theory of gases and encapsulated in the Ideal Gas Law. This direct proportionality underscores the fundamental relationship between temperature and the average speed of gas molecules.
It's important to note that while intermolecular forces are generally negligible in ideal gases, real gases exhibit some degree of intermolecular attraction, particularly at high pressures and low temperatures. These interactions can influence the behavior of real gases and deviate from the predictions of the Ideal Gas Law.
The Impact of Intermolecular Forces
Intermolecular forces significantly impact the kinetic energy and behavior of matter, especially in condensed phases (liquids and solids). These forces dictate the degree of particle movement, the energy required for phase transitions, and various macroscopic properties.
Understanding the nature and strength of intermolecular forces is crucial for comprehending the diverse characteristics observed across different states of matter. The interplay between kinetic energy and intermolecular forces governs the physical world around us, from the rigidity of a diamond to the fluidity of water and the compressibility of air.
Kinetic Energy Across Different States of Matter
Having established the fundamental link between kinetic energy and the behavior of gases, it's natural to wonder how this energy manifests itself across the three fundamental states of matter: solid, liquid, and gas. While the average kinetic energy is intrinsically linked to temperature in all states, the manifestation and influence of this energy differ considerably due to variations in intermolecular forces and particle arrangements.
Real-World Applications of Average Kinetic Energy
The theoretical understanding of average kinetic energy transcends academic exercises, grounding itself firmly in a multitude of real-world applications that shape our technological landscape. From optimizing engine efficiency to revolutionizing refrigeration techniques and understanding heat transfer processes, the implications are far-reaching and pivotal to numerous industries.
Thermodynamics: Harnessing Kinetic Energy for Efficiency
Thermodynamics, the study of energy and its transformations, relies heavily on the principles of average kinetic energy to design and optimize various systems. Understanding how kinetic energy translates into heat and work is paramount in applications like internal combustion engines and refrigeration cycles.
Engine Efficiency
In internal combustion engines, the chemical energy stored in fuel is converted into thermal energy through combustion. This thermal energy increases the average kinetic energy of the gas molecules within the cylinder, leading to a rise in temperature and pressure. The efficiency of an engine directly relates to how effectively it converts this kinetic energy into mechanical work, driving the pistons and ultimately powering the vehicle. Engineers constantly strive to optimize engine designs to maximize this conversion and minimize energy losses due to friction and heat dissipation. Sophisticated simulations and experimental analyses, guided by principles of average kinetic energy, help achieve this goal.
Refrigeration
Refrigeration cycles, conversely, utilize the principles of kinetic energy to extract heat from a cold reservoir and transfer it to a hot reservoir. This process involves a refrigerant that undergoes phase changes, absorbing heat during evaporation and releasing it during condensation.
The efficiency of a refrigerator depends on the amount of heat it can remove per unit of energy input. Understanding the kinetic energy of the refrigerant molecules at different stages of the cycle is crucial for optimizing the system's performance. By carefully controlling the pressure, temperature, and flow rate of the refrigerant, engineers can maximize the heat transfer and achieve efficient cooling.
Beyond Thermodynamics: Expanding Applications
While thermodynamics represents a central application area, the principles of average kinetic energy extend far beyond.
Materials Science
In materials science, understanding the average kinetic energy of atoms within a solid is crucial for predicting its thermal properties, such as heat capacity and thermal conductivity. Materials with high thermal conductivity, for instance, possess atoms with higher average kinetic energy, allowing them to transfer heat more efficiently. This knowledge is essential for designing heat sinks, thermal insulators, and other materials with specific thermal properties.
Chemical Reactions
The rate of chemical reactions is also profoundly influenced by the kinetic energy of the reacting molecules. Reactions typically require a certain activation energy to overcome energy barriers and proceed. Increasing the temperature raises the average kinetic energy of the molecules, increasing the probability that they will collide with sufficient energy to react. This principle underlies the control of reaction rates in various industrial processes.
Heat Transfer: The Flow of Kinetic Energy
Heat transfer, the movement of thermal energy from one system to another, is intrinsically linked to the average kinetic energy of the constituent particles. Heat transfer occurs through three primary mechanisms: conduction, convection, and radiation.
Conduction
Conduction involves the transfer of heat through a material by the collision of particles with different kinetic energies. In solids, heat is conducted primarily through the vibrations of atoms within the lattice structure. In liquids and gases, heat is conducted through the collisions of molecules.
Convection
Convection involves the transfer of heat through the movement of fluids (liquids or gases). When a fluid is heated, its density decreases, causing it to rise and be replaced by cooler, denser fluid. This movement creates a convection current that transfers heat throughout the fluid.
Radiation
Radiation involves the transfer of heat through electromagnetic waves, which do not require a medium to propagate. All objects emit thermal radiation, and the amount of radiation emitted depends on the object's temperature and surface properties. The higher the temperature, the greater the average kinetic energy of the particles, and the more radiation emitted.
Understanding the relationships between average kinetic energy and these heat transfer mechanisms is essential for designing efficient heating and cooling systems, optimizing industrial processes, and understanding climate phenomena.
Video: Unlock Kinetic Energy: Simple Guide Everyone Needs!
FAQs about Understanding Kinetic Energy
Here are some common questions about kinetic energy to help you grasp the concept better. We hope these answers clarify any lingering confusion.
What exactly is kinetic energy?
Kinetic energy is simply the energy of motion. Any object that's moving, whether it's a car speeding down the highway or a tiny dust particle floating in the air, possesses kinetic energy. The faster an object moves, the more kinetic energy it has.
How is kinetic energy different from potential energy?
Potential energy is stored energy, representing the potential to do work. A stretched rubber band or a book sitting on a shelf has potential energy. When the rubber band is released or the book falls, that potential energy is converted into kinetic energy as they move.
What factors influence the amount of kinetic energy an object has?
The amount of kinetic energy depends on two key factors: the object's mass and its velocity. A heavier object moving at the same speed as a lighter object will have more kinetic energy. Similarly, an object moving faster will have more kinetic energy than the same object moving slower. These factors influence the average kinetic energy in a system or object.
Can kinetic energy be transformed into other forms of energy?
Absolutely! Kinetic energy can be readily transformed into other forms of energy, such as heat, sound, or electrical energy. For example, when you apply the brakes in a car, the kinetic energy of the car is converted into heat due to friction between the brake pads and the rotors. This transformation also affects the average kinetic energy in a system or object.