Molar Enthalpy Explained: Your Ultimate Guide!
Understanding molar enthalpy, a crucial concept in thermochemistry, becomes significantly easier when you consider its relationship to Hess's Law. Hess's Law provides the foundational principle allowing calculation of molar enthalpy changes. Furthermore, the accurate measurement of molar enthalpy often relies on techniques developed by pioneers like Antoine Lavoisier, whose contributions laid groundwork for modern calorimetry. A good grasp of molar enthalpy is essential for students and professionals alike, especially those using simulation software such as ChemCAD for process design and analysis. So, let's embark on this journey to unravel the intricacies of molar enthalpy and equip you with the knowledge you need to confidently apply it.
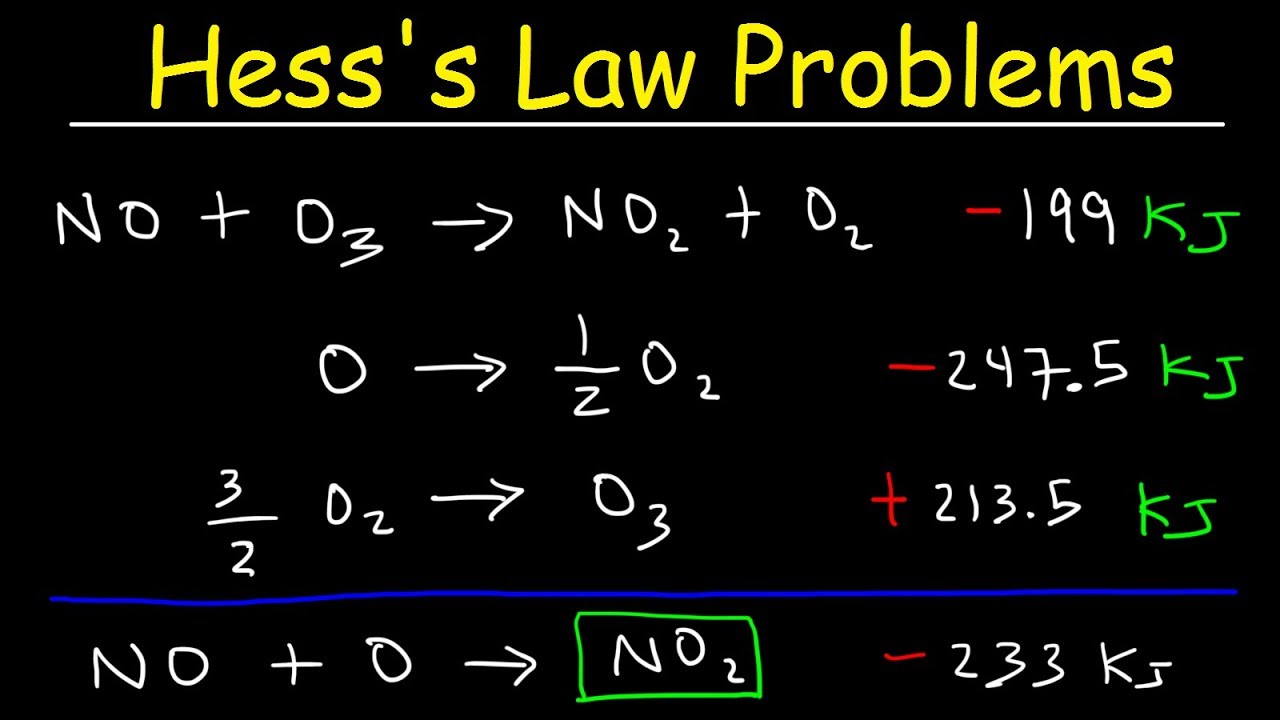
Image taken from the YouTube channel The Organic Chemistry Tutor , from the video titled Hess's Law Problems & Enthalpy Change - Chemistry .
Unveiling the Essence of Molar Enthalpy
Molar enthalpy, a cornerstone of chemical thermodynamics, reveals the intricate energy dance accompanying chemical reactions and phase transitions.
Its comprehension unlocks deeper insights into reaction feasibility, heat transfer mechanisms, and energy balances vital across scientific and engineering disciplines.
Molar enthalpy (ΔHm) represents the change in enthalpy when one mole of a substance undergoes a specific process at constant pressure.
Think of it as the energetic fingerprint of a reaction or a phase change, quantifying the heat absorbed or released per mole of reactant or product.
It is typically expressed in units of kilojoules per mole (kJ/mol).
The sign of ΔHm indicates whether the process is exothermic (releases heat, ΔHm < 0) or endothermic (absorbs heat, ΔHm > 0).
Why Understanding Molar Enthalpy Matters
Comprehending molar enthalpy is crucial for several reasons:
-
Predicting Reaction Feasibility: By knowing the molar enthalpy change of a reaction, chemists can predict whether the reaction will proceed spontaneously under given conditions.
-
Optimizing Chemical Processes: In industrial settings, understanding molar enthalpy allows for the optimization of reaction conditions to maximize product yield while minimizing energy consumption.
-
Analyzing Physical Processes: Molar enthalpy is equally important in understanding physical processes such as melting, boiling, and dissolution, each characterized by unique energy requirements.
-
Ensuring Safety: Calculating molar enthalpy changes helps assess the potential hazards associated with chemical reactions, enabling the implementation of appropriate safety measures.
Molar Enthalpy and its Link to Thermodynamics
Molar enthalpy is intrinsically linked to the broader field of thermodynamics, particularly the First Law of Thermodynamics, which states that energy is conserved.
Enthalpy, a state function, provides a convenient way to track energy changes in chemical and physical processes occurring at constant pressure.
By applying thermodynamic principles and using molar enthalpy values, scientists and engineers can make accurate predictions about the energetic behavior of systems, driving innovation and problem-solving in various domains.
Unpacking the significance of molar enthalpy requires a firm grasp of the core concept upon which it's built: enthalpy itself. Understanding enthalpy, its components, and its relationship to molar enthalpy is paramount to navigating the complexities of thermochemistry.
Defining Enthalpy: The Foundation of Molar Enthalpy
Enthalpy (H) is a thermodynamic property of a system that represents the total heat content. It's a state function, meaning its value depends only on the current state of the system. It does not depend on the path taken to reach that state.
The Enthalpy Equation: Connecting Energy, Pressure, and Volume
Enthalpy is defined by the equation: H = U + PV, where:
- H represents enthalpy.
- U represents the internal energy of the system (the energy associated with the motion and interactions of its molecules).
- P represents the pressure of the system.
- V represents the volume of the system.
This equation reveals that enthalpy accounts for both the internal energy of a system and the energy associated with the system's pressure and volume. Any change in a system's internal energy, pressure, or volume will thus impact its enthalpy.
Enthalpy vs. Molar Enthalpy: Distinguishing the Terms
While enthalpy (H) represents the total heat content of a system, molar enthalpy (ΔHm) specifically refers to the change in enthalpy when one mole of a substance undergoes a particular process at constant pressure.
Molar enthalpy is therefore an intensive property, meaning it doesn't depend on the amount of substance. It is a standardized measure, allowing for comparisons between different substances and reactions.
The Role of Moles in Molar Enthalpy Calculations
The concept of the mole (mol) is central to molar enthalpy. A mole is defined as 6.022 x 1023 entities (atoms, molecules, ions, etc.).
Molar enthalpy is expressed in units of energy per mole (typically kJ/mol), emphasizing that it represents the enthalpy change associated with that specific quantity of substance.
When calculating enthalpy changes for reactions involving multiple moles of reactants or products, the molar enthalpy must be multiplied by the appropriate stoichiometric coefficients to obtain the total enthalpy change for the reaction.
While understanding enthalpy as a state function and carefully distinguishing it from molar enthalpy sets the stage, the practical significance of enthalpy lies in its direct relationship with heat transfer, the very essence of how chemical reactions interact with their environment. This relationship allows us to quantify and predict the energy changes associated with chemical processes.
Heat and Enthalpy: An Intertwined Relationship
Heat, symbolized as q, is the transfer of thermal energy between a system and its surroundings. This transfer arises due to a temperature difference. Energy always flows from a region of higher temperature to a region of lower temperature until thermal equilibrium is reached. Understanding the nuanced role of heat is central to understanding the way energy changes during chemical or physical changes.
Heat: The Vehicle of Energy Transfer
Heat is not a state function; it is a process function.
This means that the amount of heat transferred depends on the path taken during the process, not just the initial and final states. When a system absorbs heat from its surroundings, we say that heat is added to the system (q > 0), and the process is endothermic. Conversely, when a system releases heat to its surroundings, heat is removed from the system (q < 0), and the process is exothermic.
Enthalpy Change at Constant Pressure
Under constant pressure conditions, which are common in many chemical reactions carried out in open containers, a remarkable simplification occurs: The heat absorbed or released by the system (qp) becomes equal to the change in enthalpy (ΔH). This relationship, expressed as ΔH = qp, is a cornerstone of thermochemistry.
It allows us to directly measure the enthalpy change of a reaction by simply measuring the heat exchanged with the surroundings at constant pressure. This relationship is not universally true.
It only holds when the pressure remains constant during the process.
Systems, Surroundings, and Heat Transfer
To accurately describe heat transfer, it's essential to define the system and its surroundings:
-
The system is the specific part of the universe that we are interested in studying.
This could be a chemical reaction taking place in a flask, a cell, or any defined region.
-
The surroundings encompass everything else in the universe outside the system.
The surroundings are capable of exchanging energy and/or matter with the system.
Heat transfer always occurs across the boundary between the system and the surroundings.
By carefully defining the system and surroundings, we can track the flow of energy and determine whether a process is releasing heat into the surroundings (exothermic) or absorbing heat from the surroundings (endothermic). Measuring the heat exchange, particularly under constant pressure, allows us to quantify the enthalpy change (ΔH) and gain valuable insights into the energy landscape of chemical reactions.
Under constant pressure conditions, the heat exchanged by a system directly reflects its change in enthalpy. This provides a crucial link between theoretical concepts and measurable quantities. But how do we establish a common ground, a starting point for comparing the enthalpy changes of different reactions and substances?
Standard Enthalpy of Formation: The Reference Point
The concept of standard enthalpy of formation (ΔHf°) provides this essential reference point. It serves as a cornerstone for thermochemical calculations, enabling us to predict and understand the energy changes associated with chemical reactions.
Defining Standard Enthalpy of Formation (ΔHf°)
The standard enthalpy of formation (ΔHf°) is defined as the enthalpy change when one mole of a compound is formed from its constituent elements in their standard states under standard conditions.
Standard conditions are defined as 298 K (25 °C) and 1 atm pressure. The standard state of an element is its most stable form under these conditions. For example, the standard state of oxygen is O2(g), and the standard state of carbon is graphite (C(s, graphite)).
It's crucial to remember these conditions when interpreting and using ΔHf° values.
Why is ΔHf° a Useful Reference?
The standard enthalpy of formation acts as a benchmark, allowing us to compare the relative stabilities of different compounds. A highly negative ΔHf° indicates that a compound is more stable than its constituent elements. This means a significant amount of energy is released during its formation. Conversely, a positive ΔHf° suggests that the compound is less stable. It requires energy input to form it from its elements.
Because the enthalpy of formation of an element in its standard state is defined as zero, this becomes the ultimate reference point. This convention simplifies calculations and provides a consistent baseline.
Calculating Enthalpy Changes of Reactions Using ΔHf°
Standard enthalpies of formation are instrumental in calculating the enthalpy change (ΔH°) for any chemical reaction under standard conditions. The principle behind this calculation stems from Hess's Law. Hess's Law states that the enthalpy change of a reaction is independent of the path taken.
The standard enthalpy change of a reaction (ΔH°rxn) can be calculated using the following equation:
ΔH°rxn = ΣnΔHf°(products) - ΣnΔHf°(reactants)
Where:
- Σ represents the summation.
- n is the stoichiometric coefficient of each substance in the balanced chemical equation.
- ΔHf°(products) is the standard enthalpy of formation of each product.
- ΔHf°(reactants) is the standard enthalpy of formation of each reactant.
This equation essentially states that the enthalpy change of a reaction is the difference between the sum of the enthalpies of formation of the products and the sum of the enthalpies of formation of the reactants.
Examples of Common Compounds and Their ΔHf°
The standard enthalpies of formation for numerous compounds have been experimentally determined and are readily available in thermodynamic tables. Here are a few examples:
- H2O(l): ΔHf° = -285.8 kJ/mol
- CO2(g): ΔHf° = -393.5 kJ/mol
- CH4(g): ΔHf° = -74.8 kJ/mol
- NH3(g): ΔHf° = -46.1 kJ/mol
These values indicate the amount of heat released (negative ΔHf°) or absorbed (positive ΔHf°) when one mole of each compound is formed from its elements in their standard states.
Understanding and utilizing standard enthalpies of formation is crucial for predicting the heat released or absorbed in chemical reactions. They are a fundamental tool in thermochemistry and have wide-ranging applications in various scientific and engineering disciplines.
But it's not always feasible or practical to directly measure the enthalpy change for every reaction. This is where the genius of Hess's Law comes into play, offering an elegant workaround for calculating enthalpy changes indirectly.
Hess's Law: Calculating Enthalpy Changes Indirectly
Hess's Law is a fundamental principle in thermochemistry that allows us to determine the enthalpy change (ΔH) for a reaction without directly measuring it. This is particularly useful for reactions that are difficult or impossible to perform in a calorimeter.
Enthalpy as a State Function
At the heart of Hess's Law lies the concept of enthalpy as a state function. A state function is a property that depends only on the initial and final states of a system, regardless of the path taken to get there.
Think of climbing a mountain. The total change in altitude is the same whether you take a direct route straight up or a winding trail around the mountain.
Similarly, the enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
This independence from the reaction pathway is what makes Hess's Law so powerful.
The Principle of Hess's Law
Hess's Law states that the enthalpy change for a reaction is the sum of the enthalpy changes for each step in the reaction, regardless of the number of steps.
In other words, if a reaction can be broken down into a series of steps, the overall enthalpy change is simply the sum of the enthalpy changes for each individual step.
Mathematically, this can be expressed as:
ΔHreaction = ΔH1 + ΔH2 + ΔH3 + ...
Applying Hess's Law: A Step-by-Step Approach
Using Hess's Law involves manipulating known enthalpy changes of reactions to calculate the enthalpy change of a target reaction. Here's a step-by-step guide:
-
Identify the Target Reaction: Clearly define the reaction for which you want to determine the enthalpy change.
-
Gather Relevant Reactions: Find a set of reactions with known enthalpy changes that, when combined, will yield the target reaction. These are often called "intermediate" reactions.
-
Manipulate the Reactions: This is the crucial step. You may need to do the following:
- Reverse a Reaction: If a reactant or product is on the wrong side of the equation compared to the target reaction, reverse the reaction. When you reverse a reaction, change the sign of ΔH.
- Multiply a Reaction: If you need to adjust the stoichiometric coefficients to match the target reaction, multiply the entire reaction (including the ΔH) by the appropriate factor.
-
Cancel Common Species: After manipulating the reactions, add them together. Cancel out any species that appear on both sides of the equation (reactants and products).
-
Calculate the Overall ΔH: Add the enthalpy changes for the manipulated reactions. The result is the enthalpy change for the target reaction.
Examples of Applying Hess's Law
Let's illustrate Hess's Law with a classic example:
Example 1: Calculating the Enthalpy of Formation of Methane (CH4)
We want to find the enthalpy change for the following reaction:
C(s, graphite) + 2H2(g) → CH4(g) ΔH = ?
We are given the following reactions with known enthalpy changes:
- C(s, graphite) + O2(g) → CO2(g) ΔH1 = -393.5 kJ
- H2(g) + ½O2(g) → H2O(l) ΔH2 = -285.8 kJ
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔH3 = -890.4 kJ
Solution:
-
Reverse Reaction 3: We need CH4 on the product side, so we reverse the third reaction and change the sign of ΔH3:
CO2(g) + 2H2O(l) → CH4(g) + 2O2(g) ΔH3' = +890.4 kJ
-
Multiply Reaction 2 by 2: We need 2 moles of H2, so we multiply the second reaction by 2:
2H2(g) + O2(g) → 2H2O(l) ΔH2' = 2
**(-285.8 kJ) = -571.6 kJ
-
Add the Reactions: Now we add the first reaction, the modified second reaction, and the reversed third reaction:
C(s, graphite) + O2(g) → CO2(g) ΔH1 = -393.5 kJ 2H2(g) + O2(g) → 2H2O(l) ΔH2' = -571.6 kJ CO2(g) + 2H2O(l) → CH4(g) + 2O2(g) ΔH3' = +890.4 kJ
C(s, graphite) + 2H2(g) → CH4(g)
-
Calculate the Overall ΔH: Add the enthalpy changes:
ΔH = ΔH1 + ΔH2' + ΔH3' ΔH = -393.5 kJ + (-571.6 kJ) + 890.4 kJ ΔH = -74.7 kJ
Therefore, the enthalpy change for the formation of methane is -74.7 kJ.
Example 2: A More Complex Scenario
Hess's Law shines when dealing with reactions that are difficult to perform directly.
Consider calculating the enthalpy change for the formation of sulfur trioxide (SO3) from sulfur and oxygen:
2S(s) + 3O2(g) → 2SO3(g) ΔH = ?
Suppose we only have the following data:
- S(s) + O2(g) → SO2(g) ΔH1 = -296.8 kJ
- 2SO2(g) + O2(g) → 2SO3(g) ΔH2 = -197.8 kJ
Solution:
-
Multiply Reaction 1 by 2: To get 2 moles of S(s) on the reactant side, multiply the first reaction by 2:
2S(s) + 2O2(g) → 2SO2(g) ΔH1' = 2** (-296.8 kJ) = -593.6 kJ
-
Add the Reactions: Now add the modified first reaction and the second reaction:
2S(s) + 2O2(g) → 2SO2(g) ΔH1' = -593.6 kJ 2SO2(g) + O2(g) → 2SO3(g) ΔH2 = -197.8 kJ
2S(s) + 3O2(g) → 2SO3(g)
-
Calculate the Overall ΔH: Add the enthalpy changes:
ΔH = ΔH1' + ΔH2 ΔH = -593.6 kJ + (-197.8 kJ) ΔH = -791.4 kJ
Therefore, the enthalpy change for the formation of 2 moles of SO3 is -791.4 kJ.
Hess's Law is a cornerstone of thermochemical calculations. It enables us to:
-
Calculate enthalpy changes for reactions that are difficult or impossible to measure directly.
-
Determine the stability of compounds relative to their constituent elements.
-
Predict the heat released or absorbed in chemical reactions, which is crucial for designing and optimizing chemical processes.
By understanding and applying Hess's Law, we gain a deeper insight into the energetic changes that accompany chemical reactions and harness this knowledge for various practical applications.
Hess's Law provides a powerful theoretical framework, but ultimately, we need experimental methods to anchor our understanding of enthalpy changes to the real world. The direct measurement of heat transfer during chemical and physical processes relies on a technique called calorimetry, the topic we'll explore in this section.
Calorimetry: Measuring Enthalpy Changes Experimentally
Calorimetry is the science of measuring heat flow associated with chemical reactions or physical changes. It’s the experimental cornerstone that allows us to quantify enthalpy changes (ΔH) and gain insights into the energy dynamics of various processes.
The Essence of Calorimetry
At its core, calorimetry involves monitoring the temperature change of a known mass of a substance (often water) when a reaction occurs within a controlled environment. By carefully measuring this temperature change and knowing the heat capacity of the substance, we can calculate the amount of heat absorbed or released during the reaction.
This direct measurement of heat provides the crucial link between theoretical enthalpy calculations and experimental observations.
The Calorimeter: A Controlled Environment
A calorimeter is an insulated container designed to minimize heat exchange with the surroundings. This isolation is critical for accurate measurements, as it ensures that virtually all the heat released or absorbed by the reaction is transferred to the calorimeter's contents (usually water).
Basic Components of a Calorimeter
-
Insulated Container: This minimizes heat exchange with the environment, crucial for accurate measurements.
-
Reaction Chamber: Where the chemical reaction or physical process takes place.
-
Thermometer: Used to precisely measure the temperature change of the calorimeter's contents.
-
Stirrer: Ensures uniform temperature distribution throughout the water.
Units of Energy: Joules and Kilojoules
Heat and enthalpy changes are forms of energy, and are therefore measured in Joules (J) in the International System of Units (SI). Since chemical reactions often involve significant amounts of energy, it's common to express enthalpy changes in Kilojoules (kJ), where 1 kJ = 1000 J.
These units provide a standardized way to quantify the energy released or absorbed during a chemical process, enabling comparisons between different reactions and processes.
Heat Capacity: The Key to Calorimetry Calculations
Heat capacity (C) is a fundamental property of a substance that describes the amount of heat required to raise its temperature by one degree Celsius (or one Kelvin). In calorimetry, heat capacity plays a vital role in calculating the heat absorbed or released by the calorimeter's contents.
Specific Heat Capacity
A related term, specific heat capacity (c), refers to the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius (or one Kelvin). Water, often used in calorimeters, has a high specific heat capacity, making it an effective heat absorber.
The Calorimetry Equation
The relationship between heat (q), mass (m), specific heat capacity (c), and temperature change (ΔT) is expressed by the equation:
q = mcΔT
This equation is the cornerstone of calorimetry calculations, allowing us to determine the amount of heat transferred based on the measured temperature change and known properties of the calorimeter's contents. By meticulously applying this equation and understanding the principles of heat capacity, we can accurately measure enthalpy changes and gain valuable insights into the energetic nature of chemical reactions and physical processes.
Hess's Law provides a powerful theoretical framework, but ultimately, we need experimental methods to anchor our understanding of enthalpy changes to the real world. The direct measurement of heat transfer during chemical and physical processes relies on a technique called calorimetry, the topic we'll explore in this section.
Exothermic and Endothermic Reactions: The Heat Exchange
Chemical reactions are fundamentally about energy transformation. Some reactions release energy into their surroundings, while others absorb energy from them. These two categories, known as exothermic and endothermic reactions, dictate the flow of heat and are characterized by the sign of their enthalpy change (ΔH).
Defining Exothermic and Endothermic Reactions
An exothermic reaction is one that releases heat into the surroundings. Think of a burning log, the explosion of dynamite, or the setting of cement. In these processes, the energy released is usually in the form of heat, light, or sound.
Conversely, an endothermic reaction absorbs heat from its surroundings. Examples include melting ice, photosynthesis, or the dissolving of ammonium nitrate in water. These reactions require a continuous input of energy to proceed.
Enthalpy Changes (ΔH): The Thermochemical Signature
The enthalpy change (ΔH) is the key indicator of whether a reaction is exothermic or endothermic. Enthalpy, remember, is a measure of the total heat content of a system at constant pressure.
- For exothermic reactions, ΔH is negative (ΔH < 0). This is because the system loses heat to the surroundings. The products have lower enthalpy than the reactants.
- For endothermic reactions, ΔH is positive (ΔH > 0). Here, the system gains heat from the surroundings. The products have higher enthalpy than the reactants.
The magnitude of ΔH indicates the amount of heat released or absorbed per mole of reaction. A large negative ΔH signifies a highly exothermic reaction, whereas a large positive ΔH represents a highly endothermic reaction.
Real-World Examples
Understanding exothermic and endothermic reactions is critical because they are ubiquitous in our daily lives and in industrial processes.
Exothermic Examples
- Combustion: Burning fuel (wood, propane, natural gas) is a classic example. The reaction releases heat and light, making it useful for heating and power generation.
- Neutralization: The reaction between an acid and a base (e.g., hydrochloric acid and sodium hydroxide) releases heat, forming salt and water.
- Thermite Reaction: This reaction between a metal oxide (e.g., iron oxide) and a metal (e.g., aluminum) generates intense heat. It is used in welding and demolition.
Endothermic Examples
- Photosynthesis: Plants absorb sunlight (energy) to convert carbon dioxide and water into glucose and oxygen. This process stores energy in the form of chemical bonds.
- Melting Ice: Ice absorbs heat from the surroundings to transition from a solid to a liquid state.
- Electrolysis of Water: Passing an electric current through water breaks it down into hydrogen and oxygen. This requires a continuous input of electrical energy.
Understanding the distinction between exothermic and endothermic reactions is fundamental to thermochemistry and provides insights into energy transformations in chemical processes. The sign and magnitude of ΔH provide a quantitative measure of the heat exchange, allowing us to predict and control chemical reactions for a wide range of applications.
Bond Enthalpy: A Tool for Estimating Enthalpy Changes
Having explored calorimetry and its direct measurement of heat, we now turn to a more theoretical approach: using bond enthalpies to estimate enthalpy changes. This method leverages the relationship between chemical bonds and energy, offering a valuable shortcut for predicting reaction energetics.
Understanding Bond Enthalpy
Bond enthalpy, also known as bond dissociation energy, is defined as the amount of energy required to break one mole of a specific bond in the gas phase.
This value is always positive, as energy must be input to overcome the attractive forces holding the atoms together.
Each chemical bond has a characteristic bond enthalpy. For example, the bond enthalpy of a single C-H bond is different from that of a C=O double bond. These values are typically reported in units of kJ/mol.
Estimating Enthalpy Changes Using Bond Enthalpies
The fundamental principle behind using bond enthalpies is that breaking bonds requires energy (endothermic), while forming bonds releases energy (exothermic).
To estimate the enthalpy change (ΔH) of a reaction, we can use the following conceptual equation:
ΔH ≈ Σ(Bond enthalpies of bonds broken) - Σ(Bond enthalpies of bonds formed)
This equation essentially states that the enthalpy change of a reaction is approximately equal to the sum of the energies required to break all the bonds in the reactants, minus the sum of the energies released when all the bonds in the products are formed.
Here's a step-by-step approach to estimate ΔH using bond enthalpies:
- Draw the Lewis structures of all reactants and products to identify all the bonds present.
- List all the bonds broken in the reactants, along with their corresponding bond enthalpies. Sum these values.
- List all the bonds formed in the products, along with their corresponding bond enthalpies. Sum these values.
- Apply the equation: ΔH ≈ Σ(Bonds broken) - Σ(Bonds formed).
- Pay close attention to stoichiometric coefficients. If a molecule has a coefficient of 2, then all of its bond energies must be multiplied by 2.
Example: Consider the simple reaction: H₂(g) + Cl₂(g) → 2HCl(g)
- Bonds broken: 1 mol H-H, 1 mol Cl-Cl.
- Bonds formed: 2 mol H-Cl.
- ΔH ≈ [BE(H-H) + BE(Cl-Cl)] - [2 * BE(H-Cl)].
- Using typical bond enthalpy values (available in reference tables), we can calculate an approximate ΔH for this reaction.
Limitations of Bond Enthalpies
While bond enthalpies offer a convenient way to estimate enthalpy changes, it's crucial to recognize their limitations:
- Averaged Values: Bond enthalpies are average values derived from a range of compounds. The actual bond enthalpy in a specific molecule can vary depending on the molecular environment.
- Gas Phase: Bond enthalpies are defined for the gas phase. If reactants or products are in liquid or solid phases, additional energy considerations (such as enthalpy of vaporization or fusion) are needed.
- Resonance: Molecules exhibiting resonance (delocalization of electrons) can have bond strengths that deviate significantly from typical bond enthalpy values.
- Ionic Character: Bonds with significant ionic character may not be accurately represented by simple bond enthalpy values.
Due to these factors, enthalpy changes calculated using bond enthalpies are estimates, not precise measurements. More accurate methods, such as using standard enthalpies of formation or calorimetry, are preferred when precise values are required.
Despite these limitations, bond enthalpies provide a valuable tool for quickly estimating the exothermicity or endothermicity of a reaction and for gaining a conceptual understanding of the energy changes associated with bond breaking and formation. They serve as a useful approximation in many chemical contexts.
The Ideal Gas Law and its Limited Connection to Enthalpy
While we've explored various methods for calculating enthalpy changes, from direct calorimetric measurements to estimations using bond enthalpies, it's important to understand the role of the Ideal Gas Law and its connection, albeit limited, to enthalpy.
The Ideal Gas Law, a cornerstone of chemistry and physics, provides a simplified model for the behavior of gases under certain conditions. Understanding its relationship to enthalpy requires careful consideration.
A Quick Review of the Ideal Gas Law
The Ideal Gas Law is expressed as:
PV = nRT
Where:
- P represents the pressure of the gas.
- V represents the volume of the gas.
- n represents the number of moles of the gas.
- R represents the ideal gas constant.
- T represents the absolute temperature of the gas (in Kelvin).
This equation fundamentally links the pressure, volume, and temperature of an ideal gas with the amount of substance present. It's important to remember that this law assumes that gas particles have negligible volume and no intermolecular interactions.
The Link Between the Ideal Gas Law and Enthalpy
The connection between the Ideal Gas Law and enthalpy emerges when considering the definition of enthalpy itself:
H = U + PV
Where:
- H is enthalpy.
- U is internal energy.
- P is pressure.
- V is volume.
For an ideal gas undergoing a process at constant pressure, we can express the change in enthalpy (ΔH) as:
ΔH = ΔU + PΔV
Furthermore, if the temperature changes, and assuming ideal gas behavior, PΔV can be related to nRT.
For a process involving only PV work, it can be shown that the change in internal energy, ΔU is related to the change in temperature, ΔT.
Limitations and Considerations
However, the relationship between the Ideal Gas Law and enthalpy has significant limitations:
- Ideal Gas Assumption: The Ideal Gas Law is an approximation. Real gases deviate from ideal behavior, especially at high pressures and low temperatures, where intermolecular forces become significant.
- Constant Pressure Requirement: The direct relationship between ΔH and PΔV is most straightforward at constant pressure. In processes where pressure varies, the connection becomes more complex.
- Phase Changes: The Ideal Gas Law strictly applies to gases. It cannot be directly used to calculate enthalpy changes associated with phase transitions (e.g., melting, boiling), which involve significant changes in intermolecular interactions.
When to Consider the Ideal Gas Law in Enthalpy Calculations
Despite these limitations, the Ideal Gas Law can be useful in specific scenarios:
- Estimating Volume Changes: If a reaction involves gases and you need to estimate the volume change at a specific temperature and pressure, the Ideal Gas Law can provide a reasonable approximation, provided the gases behave ideally.
- Relating ΔU and ΔH: For ideal gases at constant pressure, the Ideal Gas Law allows relating the change in internal energy (ΔU) to the change in enthalpy (ΔH), facilitating certain thermodynamic calculations.
In summary, while the Ideal Gas Law offers a valuable tool for understanding gas behavior, its connection to enthalpy is limited by the assumptions of ideal gas behavior and the conditions under which the process occurs. It’s essential to recognize these limitations and apply the Ideal Gas Law judiciously when estimating or calculating enthalpy changes.
Practical Applications of Molar Enthalpy
The theoretical understanding of molar enthalpy gains significant value when applied to real-world scenarios. Its implications resonate across diverse fields, influencing processes, designs, and safety protocols. Let's explore some key areas where molar enthalpy plays a crucial role.
Industrial Chemistry: Optimizing Reactions and Processes
In the realm of industrial chemistry, molar enthalpy is a cornerstone for optimizing chemical reactions and industrial processes. Chemical engineers meticulously analyze enthalpy changes to enhance efficiency and reduce energy consumption.
Reactor Design and Optimization
Understanding the enthalpy change of a reaction is critical in reactor design. Exothermic reactions release heat and may require cooling systems to prevent overheating and potential hazards. Conversely, endothermic reactions absorb heat and may need external heating to maintain reaction rates.
Molar enthalpy data helps engineers calculate the amount of heat that needs to be added or removed, enabling them to design reactors that operate safely and efficiently.
Process Efficiency and Energy Consumption
Minimizing energy consumption is a primary goal in industrial chemistry. By accurately determining the enthalpy changes associated with each step of a chemical process, engineers can identify opportunities to recover and reuse heat.
This is particularly important in large-scale operations, where even small improvements in energy efficiency can lead to significant cost savings and reduced environmental impact. Optimizing a large industrial plant can be streamlined and managed more effectively by understanding and controlling the heat exchanges.
Environmental Science: Assessing Environmental Impact
Molar enthalpy plays a vital role in environmental science, particularly in understanding and mitigating the environmental impact of various processes.
Combustion and Emissions Analysis
The combustion of fuels, a significant source of energy, also contributes to air pollution. Molar enthalpy calculations are used to determine the heat released during combustion and to assess the efficiency of different fuels.
This information is crucial for developing cleaner burning technologies and reducing greenhouse gas emissions. Understanding the complete combustion process, including the enthalpy changes, is key.
Waste Management and Treatment
Many waste treatment processes involve chemical reactions, and molar enthalpy can provide insights into the energy requirements and potential hazards associated with these processes.
For instance, incineration, a common method for waste disposal, releases heat. Determining the molar enthalpy of combustion for various waste materials can help optimize incinerator design and minimize emissions.
Safety Assessments: Hazard Analysis and Mitigation
Molar enthalpy is essential for evaluating the safety of chemical processes and preventing accidents.
Identifying Potential Hazards
Reactions with large enthalpy changes, whether exothermic or endothermic, pose potential hazards. Exothermic reactions can lead to runaway reactions, explosions, or fires. Endothermic reactions, while less immediately dangerous, can lead to equipment failure or the formation of hazardous byproducts.
Molar enthalpy data allows safety engineers to identify and mitigate these risks by implementing appropriate control measures.
Designing Safety Systems
Based on the potential hazards identified through molar enthalpy analysis, engineers can design safety systems such as pressure relief valves, emergency cooling systems, and fire suppression systems. These systems help prevent accidents and protect personnel and equipment.
Video: Molar Enthalpy Explained: Your Ultimate Guide!
Molar Enthalpy Explained: FAQs
Here are some frequently asked questions about molar enthalpy and how it's used.
What exactly is molar enthalpy?
Molar enthalpy is the change in enthalpy when one mole of a substance undergoes a specific process at constant pressure. It's essentially the enthalpy change per mole. Common examples are molar enthalpy of formation, combustion, and vaporization.
How is molar enthalpy different from standard enthalpy change?
Standard enthalpy change refers to the enthalpy change when a reaction occurs under standard conditions (usually 298 K and 1 atm) with all reactants and products in their standard states. Molar enthalpy refers to the enthalpy change of a specific process for just one mole. Standard enthalpy changes may incorporate molar enthalpies.
Why is molar enthalpy useful?
Molar enthalpy allows for easy comparison of enthalpy changes between different substances or reactions, since it's normalized to a per-mole basis. You can easily calculate the total enthalpy change for a reaction involving multiple moles by multiplying the molar enthalpy by the number of moles involved.
Can molar enthalpy be negative?
Yes, molar enthalpy can be negative. A negative molar enthalpy indicates an exothermic process, meaning heat is released to the surroundings when one mole of the substance undergoes that specific process. Combustion reactions, for example, often have negative molar enthalpies.
Alright, that wraps up our exploration of molar enthalpy! Hopefully, everything's a bit clearer now. Give those calculations a try and see what you can do. Happy experimenting!
